Canadian Medstore Improve Clinical Outcome
Nanoparticle Design and Development
The ability to optimize drug loading, particle size, particle components, conjugation linker chemistry and formulation process leads to tailored nanoparticles that have superior biological properties compared to the free drug they contain. The physical-chemical properties of the nanoparticle, such as surface charge and surface composition, affect the distribution of the particle in the body and are used to passively target the nanoparticle into target tissues. The composition and linker chemistry are chosen to optimize the release of the free drug, achieving a much improved pharmacokinetic profile and reducing systemic exposure. During the design of the nanoparticle, scale-up and manufacturing are considered, ensuring the production of nanoparticle populations of consistent physical characteristics at scale.
Cerulean has access to two complementary technologies within its nanoparticle platform. Both of these technologies provide for the formation of stable and consistent nanoparticles comprised of biocompatible components, which have high drug loading capacity, and are able to release active drug in a controlled fashion.
The Polymeric Nanoparticle Technology (PNP) allows for nanoparticle customization through conjugation chemistry, particle composition, formulation, and fine-tuning of nanoparticle size. This technology has produced product candidates with compelling pre-clinical validation.
The Cyclodextrin Nanoparticle Technology (CDP) produces self-assembled nanoparticles of a defined size range. The most advanced product CRLX101 (formerly IT-101) from this technology is presently in Phase 2a clinical development. This technology has been applied to several classes of molecules.
Biological Impact
When the Cerulean nanoparticles are administered in the bloodstream, they maintain their integrity in circulation, minimizing non-specific systemic drug dissemination and clearance. The size and surface properties of the nanoparticles favor extravasation through leaky vasculature, followed by deep penetration and retention at the tumor site. The physical properties of the nanoparticles facilitate endocytic intracellular uptake and help to avoid multi-drug resistance as the nanoparticles are not substrates of multi-drug transporters. The polymer-drug conjugation chemistry provides for controlled and sustained drug release, maximizing drug exposure to tumor target cells. The nanoparticles are made of biocompatible building blocks that allow for safe administration and excretion.
Our nanoparticles produce significant and prolonged tumor growth delay and improved tolerability compared to free drug in multiple mouse cancer models. Favorable drug distribution supports enhanced nanoparticle localization to the tumor and the improved pharmacokinetic profile demonstrate the advantages provided by the nanoparticles such as sustained release and shielding of the active drug.
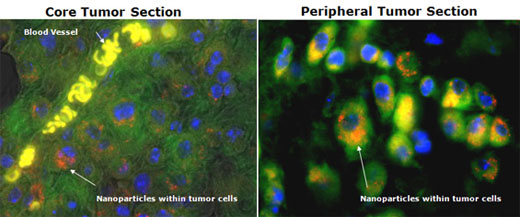
Below is an example of nanoparticles produced with our PNP technology penetrating deep into tumor tissue and far from blood vessels, co-localizing with endosomes and demonstrating that these nanoparticles enter tumor cells as intact nanoparticles.
CRLX288 was developed with Cerulean’s proprietary PEGylated polymeric nanoparticle technology (PNP) with the goal of further improving the therapeutic index of docetaxel. CRLX288 was designed by conjugating docetaxel to the biodegradable polymer poly (lactic-co-glycolic acid) and forming nanoparticles by nanoprecipitation. Mouse pharmacokinetic and biodistribution data demonstrate that CRLX288 has prolonged circulation time and enhanced tumor localization compared to the parent drug docetaxel, as evidenced by both half-life and Area Under the Curve values.
Such improved pharmacokinetics resulted in enhanced drug retention in tumor tissues. Results from tumor growth delay studies of CRLX288 illustrate that our PNP technology confers a higher maximum-tolerated dose, dramatically superior efficacy, and a longer dosing interval compared to the parent drug docetaxel. Confocal microscopy confirms that the improved efficacy and tolerability of CRLX288 nanopharmaceutical formulation is mediated by enhanced tumor penetration, and intracellular uptake and release of the parent drug in tumor cells, resulting in prolonged and sustained drug exposure. These finding with CRLX288 illustrate that the PNP platform is a powerful nanopharmaceutical technology platform capable of maximizing the therapeutic value of a broadly used pharmaceutical product, creating potentially unprecedented therapeutic opportunities for patients.
The nanopharmaceutical design is intended to improve clinical outcome by making docetaxel more efficacious, enable more patients to receive full-course therapy, and allow for more versatile drug combination options.
Profound Clinical Outcome
These highly optimized Cerulean nanoparticles produce a profound clinical outcome by allowing for high and sustained therapeutic drug levels at the target tissue and, in this way, maximizing therapeutic effects. The nanoparticles are active in drug-resistant preclinical tumor models, are well tolerated, and enable full-course therapy with minimal toxicities. Because of these attributes, our nanoparticles are highly compatible agents for combination therapies and provide for more effective disease management.
CRLX101 (formerly IT-101), our lead product candidate from the CDP technology (a nanoparticle containing a highly potent topoisomerase 1 inhibitor, camptothecin) is advancing in Phase 2a studies. This nanoparticle has shown enhanced localization of drug in tumor and superior activity in 13 xenograft mouse models spanning 9 cancer types. To date, CRLX101 had demonstrated favorable safety and pharmacokinetic data in patients.